Industry 4.0, also known as the future industry, smart manufacturing, and the Industrial Internet of Things (IoT) are current phenomena essential to the growth and productivity of the industrial sector. Thus, this industrial evolution impacts the digitalization of industrial processes. The industry of the future Industry 4.0, the fourth industrial…Read more
Serialisation
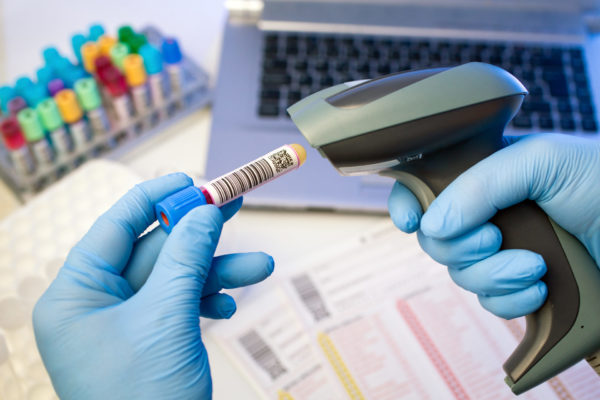
Traceability of medicines
The traceability of drugs is an important health issue in the world. What is traceability? What are the challenges of traceability? What are the means used to trace drugs? Definition of traceability There are several definitions of traceability. The first that of the ISO 9000 standard gives the following definition:…Read more

Track and trace serialization and aggregation
What is track and trace? The track trace is a system usually used for tracking postal shipments, shipping packages and objects. It is a tool for tracking the details of shipments. It is a tool that allows you to track the details of your shipments. In the pharmaceutical sector, a…Read more

Free webinar on pharmaceutical digitization | Tuesday, April 27
Pharmaceutical manufacturers, how to digitize your production operations and generate electronic batch records without disrupting your current processes? To ensure compliance with regulatory requirements, pharmaceutical manufacturers must document manual and automated procedures throughout the production process. Still very often documented on paper-based documents, their use is a source of errors,…Read more

Pharmaceutical Serialization and Aggregation
International standards for pharmaceutical products Since February 2019, the European Falsified Medicines Directive (FMD) requires laboratories and pharmacies to ensure the serialization of medicines. This involves setting up a system for verifying the authenticity of products, consisting of affixing a unique identifier to each box of medicines, which will then…Read more

Pharmaceutical products serialization
What is pharmaceutical serialization? Serialization is a system for verifying the authenticity of a drug between its release and its actual delivery to a patient. This method consists of putting a unique identifier on each box of medication in the form of a Data Matrix when it is released for…Read more

Back to the office
Our teams are gradually returning to our premises, taking all the necessary measures to ensure the health safety of all. A solid continuity plan Personal protective equipment distributed to each employee (disposable mask and hydroalcoholic gel), sustained communication, team distribution, etc., everything is done to ensure that the return is…Read more

Business continuity planning
Dear customers, dear partners, We inform you that in view of the current situation related to the spread of the virus, the Courbon Software teams have taken measures in line with national guidelines. We will no longer welcome outsiders on company premises, and will therefore favour the organization of teleworking,…Read more

inauguration of the new premises
This Friday was the inauguration of the 70, a building of more than 4,000 m² located at 70, rue de la Montat in Saint-Etienne in presence of Vincent BOUFFARD (Deputy General Manager of Vinci Energies France), Jean GALANGAU (General Manager of the Industry Center-East-Mediterranean Department) and Gaël PERDRIAU (Mayor of…Read more

